對于醫療器械CE認證企業(yè)來(lái)說(shuō)��,務(wù)必要了解歐盟是由27個(gè)成員國組成�,只有少數國家比如愛(ài)爾蘭的母語(yǔ)為英語(yǔ)���。其他國家都有自己的其他官方語(yǔ)言�。最近��,歐盟發(fā)布了指南文件��,總結了成員國的語(yǔ)言要求���,供制造商和進(jìn)口商參考�。
對于醫療器械CE認證企業(yè)來(lái)說(shuō)����,務(wù)必要了解歐盟是由27個(gè)成員國組成�,只有少數國家比如愛(ài)爾蘭的母語(yǔ)為英語(yǔ)�。其他國家都有自己的其他官方語(yǔ)言�。最近����,歐盟發(fā)布了指南文件��,總結了成員國的語(yǔ)言要求�����,供制造商和進(jìn)口商參考����。
每個(gè)國家對于醫療器械的相關(guān)文件(標簽/說(shuō)明書(shū)�,植入卡�����,EU符合性聲明��,市場(chǎng)安全通知(召回)�,符合性評價(jià)相關(guān)的文件�����,軟件的用戶(hù)界面)中使用各種語(yǔ)言��,都有自己的要求���。
請您注意:出口到對應國家的器械�,相關(guān)的文件必須使用規定的語(yǔ)言����。否則屬于不合規���,貨物在海關(guān)就有可能被阻擋����,不允許入關(guān)�����!
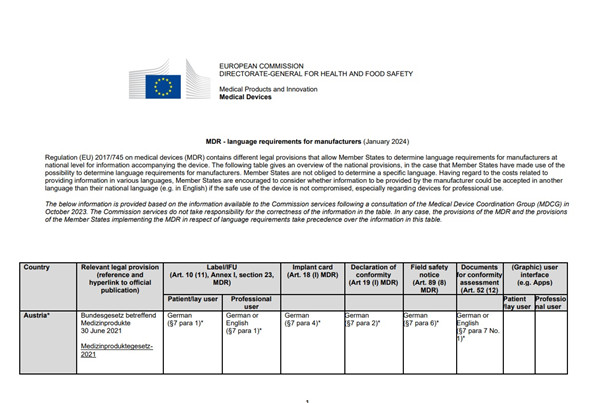
MDR - language requirements for manufacturers
(January 2024)
Regulation (EU) 2017/745 on medical devices (MDR) contains different legal provisions that allow Member States to determine language requirements for manufacturers at
national level for information accompanying the device. The following table gives an overview of the national provisions, in the case that Member States have made use of the
possibility to determine language requirements for manufacturers. Member States are not obliged to determine a specific language. Having regard to the costs related to
providing information in various languages, Member States are encouraged to consider whether information to be provided by the manufacturer could be accepted in another
language than their national language (e.g. in English) if the safe use of the device is not compromised, especially regarding devices for professional use.
The below information is provided based on the information available to the Commission services following a consultation of the Medical Device Coordination Group (MDCG) in
October 2023. The Commission services do not take responsibility for the correctness of the information in the table. In any case, the provisions of the MDR and the provisions
of the Member States implementing the MDR in respect of language requirements take precedence over the information in this table.
如有醫療器械CE認證(MDR)咨詢(xún)服務(wù)需求���,歡迎您隨時(shí)方便與杭州證標客醫藥技術(shù)咨詢(xún)有限公司聯(lián)絡(luò )����,聯(lián)系人:葉工��,電話(huà):18058734169��,微信同����。